

POLICY PERSPECTIVE
Tandem Crises: Liver Disease and Opioids
The severity of the opioid epidemic cannot be understated, but how is it linked to liver health?
Due to the horrific toll it is taking on lives, communities and our healthcare system, the opioid crisis is a great and immediate healthcare emergency. Yet, among the many reports about the opioid crisis, often referred to as an “epidemic,” little has been said about the relationship between the abuse of opioid medications and liver disease, nor about the value of opioids in pain management and treatment, especially in liver patients. Understanding and navigating effectively these off-setting circumstances will be critically important for policymakers and patients alike.
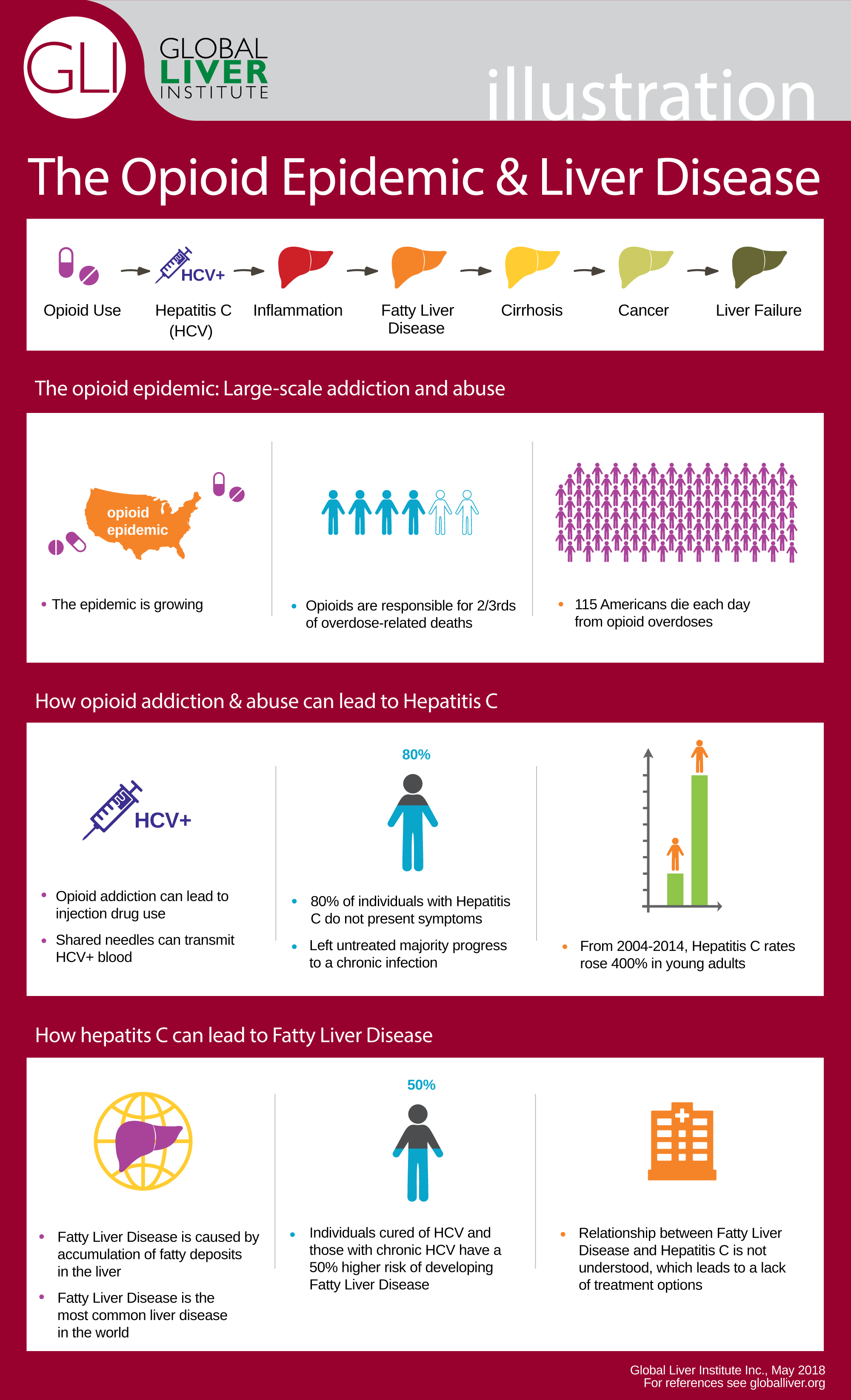
The rising rates of opioid misuse coincide with a rise in viral hepatitis, particularly in young people, and in the increase in severity of liver disease across all age groups. Rates of hepatitis C (HCV), which can be spread between opioid users injecting drugs intravenously, are skyrocketing. HCV is 5 times as infectious as HIV, for example. Chronic HCV can lead to cirrhosis, liver cancer, transplant, and eventually death. It also puts patients at higher risk for developing other liver diseases, like fatty liver disease and nonalcoholic steatohepatitis. The link between opioid use and contracting HCV has been noticed by policy makers. Bills (H.R. 5353 and S. 2579) are being introduced to fight opioid-related rises in HCV and other infectious diseases by increasing CDC funding for program implementation and expansion of surveillance and educational policies.
Although the negative impacts of the opioid crisis are startling, opioids are not simply the dangerous and terrifying medication that many believe them to be. Opioids are of extreme value in pain management, especially in patients who cannot safely take other pain medications. Many liver and gastrointestinal patients cannot take what most people refer to as “safe” medications like acetaminophen which can cause liver damage in high doses or at lower doses in already damaged livers or ibuprofen which may cause GI bleeds. In fact, for these patients, opioids may be the safer option. Opioids are extremely effective in managing pain, such as pain experienced after surgery, or pain due to severe injury and chronic pain. In patients prescribed with opioids to manage chronic pain, only about 20-30% will misuse their prescription. The other 70-80% of patients take their prescription as prescribed and rely on opioids for daily function. A critical concern is that policy responds to the opioid crisis, we need to address the unique ways that opioid abuse affects liver patients without compromising the value of opioids in treatment.
Donna Cryer, JD, President & CEO, GLI

OPEN ADVOCACY OPPORTUNITIES
Food and Drug Administration is holding its 2018 Generic Drug Regulatory Science Initiatives Public Workshop on May 24th. The workshop offers the opportunity for the public to provide input on research priorities in the area of generic drugs.
POLICY DEVELOPMENTS AT GLI
GLI’s Advanced Advocacy Academy
GLI has begun recruitment for its Advanced Advocacy Academy (A3) Class of 2018. A3 connects liver patients and family members from across the globe with the information, skills, and opportunities that they need to effectively advocate for liver health. If you or someone you know would like to attend, invite them to learn more about A3 and apply today.


FOR YOUR CALENDAR
May 18: NORD’s 35th Anniversary Celebration and 2018 Rare Impact Awards. Washington, D.C.
June 1: GLI NASH Council Meeting. Washington, D.C.
June 2-5: Digestive Disease Week. Washington, D.C.
June 2-6: 2018 American Transplant Congress. Seattle, WA.

State Action
California has sued Sutter Health, one of the state’s largest hospital networks. In the deposition Attorney General Xavier Barrera alleges that Sutter Health utilized anticompetitive practices to raise prices by setting excessively high out of network rates and restricting cost transparency information.
Colorado passed a bill (H1148) that will prohibit carriers from requiring step therapy for individuals with stage four metastatic cancer. This bill will allow providers and patients to utilize the best drug possible for a given patient’s treatment without worry of formulary coverage.
Idaho passed a bill (SB1302) that protects living organ donors from discrimination. The bill prohibits insurance companies from discriminating in the offering, cancellation, price, or conditions of an insurance policy.
Iowa passed a bill (SF2349) that will allow insurance plans into the marketplace that do not meet ACA guidelines. Their actions to provide insurance through the Iowa Farm Bureau will likely stand up to legal challenges and provide other ACA opponents with an avenue to change coverage options.
Kansas passed the Pharmacy Patients Fair Practices Act (SB351). This law allows pharmacists to discuss lower-priced alternatives with individuals without fear of penalty from pharmacy benefit managers (PBMs). It also prohibits copayments for a prescription drug from exceeding the total charges submitted by the network pharmacy, and blocks pharmacies from charging a copayment that exceeds what would have been paid if the drug had been purchased with cash. Similar bills that aim to regulate the actions of PBMs and remove gag-clauses were also passed in Arizona (HB2107), Florida (HB351), Indiana (HB1317), Kentucky (HB463), Virginia (SB933), and West Virginia (SB46). This state action has spurred federal lawmakers as well. Senator Susan Collins (R-Maine) to introduce federal legislation, the Patient Right to Know Drug Prices Act (S. 2554), which aims to regulate PBMs in a similar way.
Maryland passed a bill (HB0096) that will allow a tax deduction of up to $7,500 for expenses incurred by a living organ donor. The bill was sponsored by Speaker Michael E. Busch. Delegate Busch received a liver transplant last year after being diagnosed with late stage nonalcoholic steatohepatitis.
Maryland also passed a reinsurance program (HB1795) to help stabilize health insurance marketplaces. Reinsurance programs, commonly known as insurer’s insurance, provide assistance to insurers for extremely high claims. Minnesota and Alaska saw premiums decrease after the implementation of similar programs.
A Maryland law that targeted price gouging was overturned 2-1 by a federal appeals court. The law had given the state attorney general the power to intervene if the price of a generic drug increased by 50 percent or more in a single year. The ruling could have an adverse effect on the progress of similar legislation currently being considered in 13 other states.
Utah’s legislature voted to expand Medicaid (HB0472) for up to 70,000 additional people. However, the bill also imposes work requirements and redefines the qualifying poverty line. The bill faced pressure from an ongoing Utah ballot initiative that supports full expansion without work requirements and includes people earning up to 138 percent of the poverty line.
West Virginia passed a bill (HB4524) addressing the prescription and substitution of biologics. Under the new bill, pharmacists will have the authority to switch a patient to a less expensive interchangeable biologic unless a provider specifically notes that it is medically necessary for a patient to be on a given biological medication.

HHS NEWS
US Department of Health and Human Services (HHS)
HHS launched a new program aimed at increasing patient access to their health records. The Office of the National Coordinator for Health Information Technology (ONC) released the ONC Guide to Getting and Using your Health Records. ONC hopes to increase interoperability and general patient engagement through increased access to medical records. Interoperability, meaning that multiple providers and patients can see and use the same patient care record, is a crucial component to building patient centered and holistic care for the multidisciplinary nature of liver disease treatment. It is equally important that patients maintain control over access to their medical records.
US Food and Drug Administration (FDA)
The FDA released guidance for the effective development of Next Generation Sequencing technology, i.e., genetic- and genomic-based procedures. The statement reinforced the FDA’s commitment to keeping pace with emerging medical technologies and the importance of the continued advanced of personalized, genetic-based medicine. The support of genomic medicine is crucial to the personalized nature of liver disease and treatment.
Centers for Medicare and Medicaid Services (CMS)
A CMS proposal was released that included changes to a ruling that originally had been implemented to combat the opioid crisis. The original proposal would have limited the amount of opioids that could be prescribed. The original plan had come under scrutiny for the risk it would pose to chronically ill patients dependent on opioid medications, and the lack of clinical benefit that setting a limit would have on lowering abuse rates. Rather than setting a dosage limit, the new rule requires communication between the pharmacist and the prescriber for dosages of 90mg of morphine or more per day. This change will benefit liver patients, who often cannot safely utilize NSAIDs and other painkillers, and other chronically ill patients who depend on opioids for relief.
National Institute of Health (NIH)
NIH launched the HEAL (Helping to End Addiction Long-term) Initiative on April 4th. This effort will double funding for research into opioid misuse, addiction, and pain, and build on existing research infrastructure to find pain management alternatives. Efforts like HEAL will be essential to relieving the burden of the opioids and the concurrent epidemic of hepatitis C.
A comprehensive genetic analysis of hepatocellular carcinoma was completed by NIH. The research is part of an exhaustive genomic report on 10,000 tumors representing 33 different cancer types called The PanCancer Atlas. This represents vital progress for the continued development of personalized treatment options for cancer patients.

GRANT OPPORTUNITIES
Epidemiologic Research on Emerging Risk Factors and Liver Cancer Susceptibility (R21 Clinical Trial Not Allowed). Funds available from NIH: $200,000. Application deadlines: June 16, 2018 or October 16, 2018
Secondary Analyses in Obesity, Diabetes and Digestive and Kidney Diseases (R21 Clinical Trial Optional). Funds Available from NIH: $200,000. Application deadlines: July 16, 2018, or November, 2018
Early-Stage Preclinical Validation of Therapeutic Leads for Diseases of Interest to the NIDDK (R01). Funds available from NIH vary with project scope. Application deadlines: July 12, 2018, November 12, 2018, and March 12, 2019.
Mechanisms of Disparities in Chronic Liver Diseases and Cancer (R21). Funds available from the NIH: $275,000. Application Deadlines: April 4, 2019
NAMES TO KNOW
Daniel Best: Appointed as the new Senior Advisor for HHS drug pricing reform. In this position Best will lead initiatives aimed at lowering drug prices. Mr. Best was a former CVS Caremark executive prior to joining HHS.
Adam Boehler: Nominated as the new deputy administrator and director of the Centers for Medicare & Medicaid Innovation Center (CMMI). CMMI is responsible for the development of healthcare payment and service delivery models.
TERMS TO KNOW
Interstitium: The connective tissues and fluid compartments around the body. The interstitium plays a role in shock absorption, cell movement, our immune response, and tumor growth. It was recently recognized as a “new organ.”
Apheresis: The removal of blood plasma from the body by the withdrawal of blood, its separation into plasma and cells, and the reintroduction of the cells. Apheresis is used especially to remove antibodies in treating autoimmune diseases, and in extracorporeal liver support therapy.
Biologic: According to bio.org, “A biologic is manufactured in a living system such as a microorganism, or plant or animal cells. Most biologics are very large, complex molecules or mixtures of molecules. Many biologics are produced using recombinant DNA technology.” Check out the FDA Biologics Q&A for more information.
FURTHER READING
Do States Regret Expanding Medicaid?
The Threat of Accumulator Adjustment Programs on Patients with Rare and Chronic Diseases
The Disappearing Doctor: How Mega Mergers are Changing the Business of Health Care
