Exciting News in Liver Disease Treatment: A Milestone for MASH Patients
A Note from the Director, Dr. Sharon H. Jaycox: Advocacy, Access, and Hope for the Future
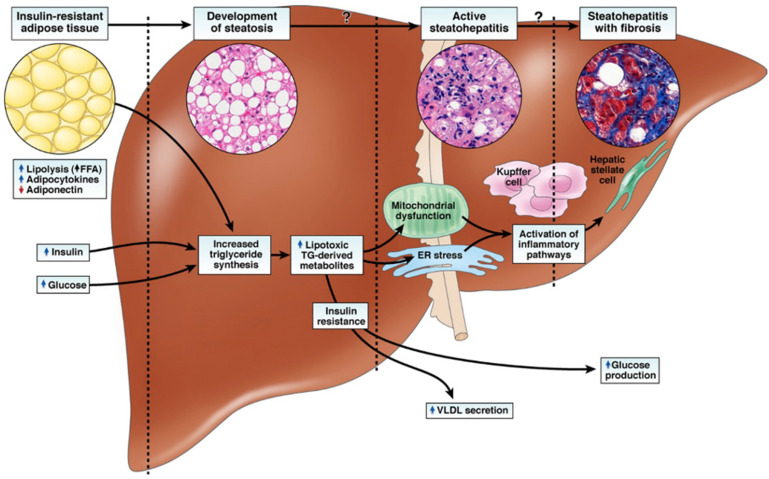
Recent developments have significantly shifted the landscape of liver disease management, delivering much-needed optimism to those affected by metabolic dysfunction-associated steatohepatitis (MASH). For years, patients and clinicians alike faced limited options, as no drug therapies had gained approval for MASH until March 2024. Now, the approval of Wegovy® (semaglutide), formerly reserved for other conditions, represents a significant leap forward, offering another therapeutic option for individuals who have long awaited a medical breakthrough. Furthermore, the European Commission’s official authorization of Rezdiffra (resmetirom), the medication already recognized in the US for treating noncirrhotic MASH with moderate to advanced liver fibrosis, expands hope across Europe, ending an era of unmet needs for patients on the continent.
Clinical trials have played a crucial role in advancing this progress, demonstrating the real-world value of research and innovation in medical care. Thanks to these trials, more patients can now benefit from therapies that can transform their lives.
While the arrival of these medications is cause for celebration, our work does not end here. We are committed to ensuring that these drugs are not only available but truly accessible for everyone who needs them. Making treatments affordable and readily obtainable remains an urgent priority.
GLI’s policy department, in collaboration with dedicated partners, stands steadfast on the front lines, advocating vigorously for our patients and their families. Together, we champion policies that break down barriers to care and fight for equitable access to life-changing treatments.
We celebrate these milestones, acknowledging the work still ahead, and invite you to join us as we continue to advocate for a healthier future for all.
Late Breaking News!
FDA Accepts Proposal for Reasonably Likely Surrogate Endpoint for ‘MASH’ all-cause Mortality or Liver-Related Events
A promising advancement for the MASH research and patient community: Progress in MASH Drug Development: the FDA’s Center for Drug Evaluation and Research (CDER) Accepts letter of intent to qualify Liver Stiffness Measurement by Vibration-Controlled Transient Elastography (VCTE) as a reasonably likely surrogate endpoint in clinical trials for adults with non-cirrhotic MASH and moderate-to-advanced liver fibrosis.
This non-invasive biomarker may:
- Predict risk of mortality or liver-related events
- Correlate with liver fibrosis severity
- Help monitor disease progression and treatment response
- Potentially reduce the need for liver biopsies in clinical trials
This supports safer, more accessible clinical trial methods, may improve trial recruitment, and could accelerate the development of much-needed therapies
This is the first step in the Drug Development Tool (DDT) qualification process, where further collaboration with the FDA will define the data and validation pathway.
Introducing GLI’s Inaugural Patient & Scientific Advisory Council! 🌟
We are proud to launch our Patient and Scientific Advisory (PSA) Council, bringing together leading patient advocates, clinicians, and researchers from across the liver health community.
This diverse group will guide GLI’s education, hashtag#advocacy, and training programs — ensuring every initiative is scientifically sound and deeply rooted in lived experience.
Thank you to our founding members for their leadership and service. Together, we’re building a stronger, more inclusive future for liver health.
Emerging Insights and Information
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Children with Obesity: An Obesity Medicine Association (OMA) and Expert Joint Perspective
Obesity among children is reaching epidemic proportions and is emerging as a critical public health concern. The intersection of obesity and MASLD forms a dangerous comorbidity that demands urgent attention from clinicians, policymakers, and society at large. As the prevalence of pediatric obesity climbs, so too does the rate of MASLD, creating a new generation at risk for chronic liver disease, metabolic syndrome, and an array of health complications that may persist into adulthood. In a review, researchers suggest lifestyle modification through obesity medication and bariatric surgery as mitigation strategies for weight reduction and MASLD progression.
Subtypes of MASLD Confer Distinct Clinical Trajectories
In a study published in the Journal of Hepatology, researchers highlighted MASLD subtypes with variations based on the underlying risk factors. Using a data-driven clustering approach, the authors identified two subtypes of MASLD, ‘cardiometabolic’ and ‘liver-specific’, which are associated with different prognostic outcomes for liver and cardiovascular disease. Both subtypes are associated with an increased risk of liver disease, but only the ‘cardiometabolic’ subtype is linked to a significantly higher risk of cardiovascular disease. These findings are consistent with earlier research indicating that higher liver fat content is associated with an elevated risk of liver-related complications, regardless of the cause.
Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes
Research has demonstrated a potential link between coffee consumption and protection against the risk of MASLD for patients with obesity and type 2 diabetes. While there is ambiguity regarding whether caffeinated or non-caffeinated coffee makes a difference in lowering the risk of MASLD, both forms of coffee contain metabolites that can reduce the severity of MASLD.
Responding to the Rise of Metabolic Liver Disease in Connecticut
It is estimated that more than one-third of residents living in Connecticut have MASLD or metabolic dysfunction-associated steatohepatitis (MASH). A working group established by the Connecticut General Assembly has been working on developing recommendations to increase public education and screening options for MASH and MASLD within the state. Some key recommendations proposed include establishing an annual Connecticut Liver Health Day (effective as of April 19, 2025), utilizing electronic health records to conduct screening events in high-risk areas using non-invasive technologies (NITs), and developing outreach programs in schools and community centers.
AI‐Based Platelet‐Independent Noninvasive Test for Liver Fibrosis in MASLD Patients
Screening for MASLD is often invasive, prompting the exploration of non-invasive testing technologies (NITs), such as platelet-based indices and imaging methods like ultrasound or MRI elastography, which offer a less intrusive alternative. Despite their advantages, NITs can be limited by cost and availability. For example, not all clinics routinely measure platelet counts, restricting the use of some platelet-based NITs. To address these challenges, researchers at Musashino Red Cross Hospital in Tokyo, Japan, developed a Support Vector Machine AI model that identifies liver fibrosis independently of platelet counts. Instead, this model utilizes accessible clinical factors, including age, sex, BMI, diabetes, hypertension, and hyperlipidemia, to facilitate broader and more practical screening.
⬇️ Tools and Resources ⬇️
Diabetes Canada Released New Guidelines for the Care and Management of MASLD in Adults with Diabetes
Comprehensive screening, timely referrals, cirrhosis surveillance, lifestyle changes, and targeted treatments help HCPs manage MASLD in type 2 diabetes (T2D) patients, leading to better health and quality of life. The Canadian consortium has recognized this and has designed guidelines to increase MASLD awareness and provide clinical recommendations for screening, diagnosis, and management in people with T2D.
Join Leading Experts at the Cardiometabolic Health Congress this October
We’re proud to announce our partnership with the 2025 Cardiometabolic Health Congress taking place October 23-25 in Boston! Join leading experts for cutting-edge research and clinical updates in cardiometabolic medicine. Use our exclusive promo code GLI100 to save $100 on your registration. Looking forward to connecting with colleagues and advancing patient care together!
Smart Tools, Healthier Livers: Digital Tools for a Healthier Liver
Digital health tools are quietly revolutionizing how we manage liver health—from scheduling doctors’ appointments to improving hepatitis B screening with culturally tailored, in-language apps. Whether you’re a patient, caregiver, or provider, these smart solutions are breaking down barriers to care and empowering people to take control of their health like never before.
In our latest blog post, “Smart Tools, Healthier Livers,” discover how innovative technologies are making liver care more accessible, personalized, and proactive.
Global Liver Institute’s Liver Health Symposium is Next Month
Global Liver Institute’s Liver Health Symposium is coming to Minneapolis, MN on Saturday, September 20, 2025—and you’re invited! This FREE, in-person event is open to both community members/patients and healthcare providers, with two distinct tracks designed to meet your needs.
For Community Members & Patients:
Learn how to protect your liver, understand your risk, and take action for your health. Hear from local healthcare experts and patient advocates, explore educational health booths, ask questions in live Q&A sessions, and receive a free liver screening.
For Healthcare Providers:
Join leading experts from Mayo Clinic and other top institutions to improve your knowledge of liver disease detection, management, and treatment. Earn free CME credits while gaining practical tools to improve patient care and participate in a provider-only workgroup focused on advancing liver care delivery.
Don’t miss this opportunity to get informed, empowered, and connected.
Upcoming Events
- September 5-8, 2025: Global Liver Institute’s Advanced Advocacy Academy (A3), Washington, D.C.
- September 11 & 12: Paris MASH Meeting, Paris, France
- September 20: Liver Health Symposium, Minnesota
- September 29 – October 1: 9th Mash Drug Development Summit, Boston, MA