Let’s Ensure Access to Treatment for PBC Patients – Pediatric & Rare Liver Diseases News
Amplifying the rare liver disease voice for PBC patients during our monthly policy call
Global Liver Institute’s August Pediatric and Rare Liver Diseases monthly council policy call gathered stakeholders from around the globe to talk about policies and regulations that affect everyone in the pediatric and rare liver space. This month’s agenda had a special focus on those with primary biliary cholangitis (PBC).
With advancements in second-line treatments for PBC in 2024, both current and new treatment options must remain available, so that every patient can choose the treatment that best meets their needs. To emphasize this, Gema Iribar of Albi España (and powerful, international PBC advocate) kicked off the meeting by sharing the patient perspective. She also encouraged rare liver disease organizations to make our voices less rare by engaging collectively with regulators on this issue of access!
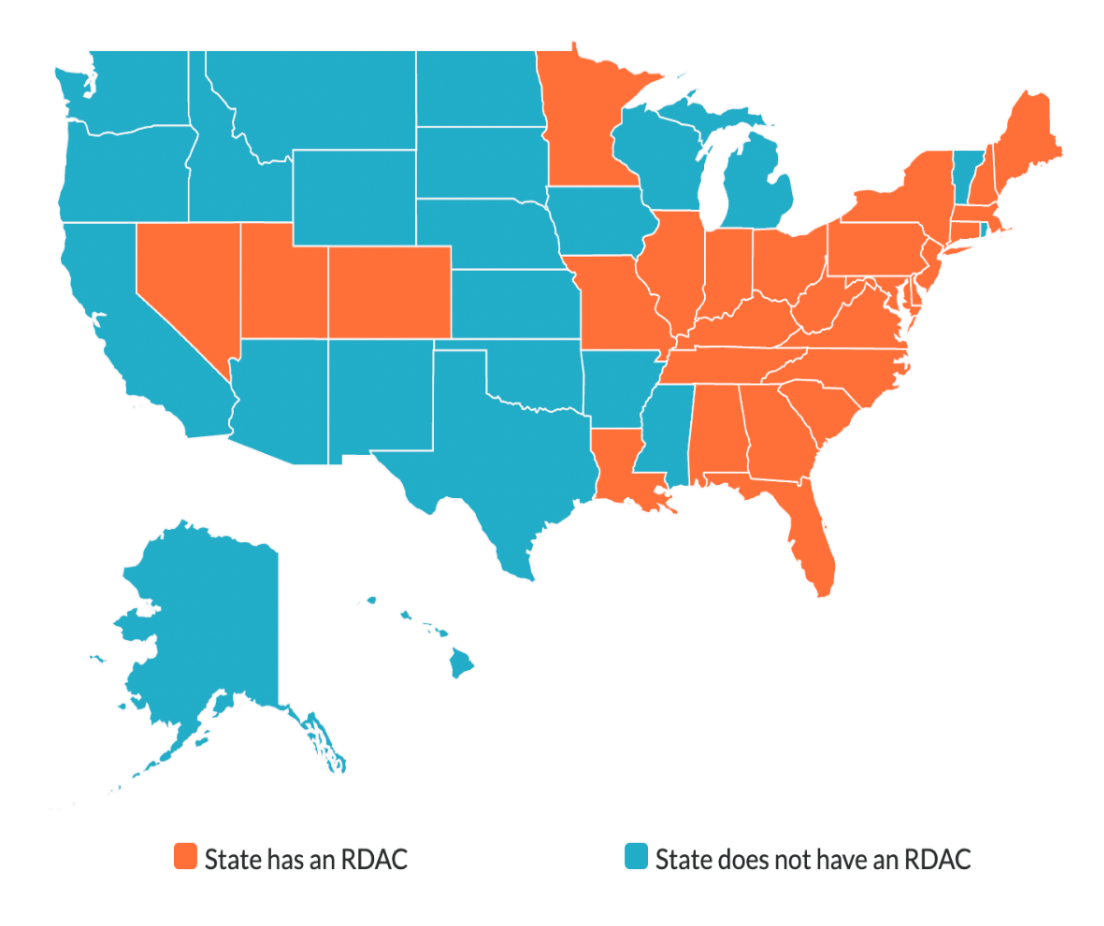
Additionally, the call reviewed ongoing efforts to collaborate with Rare Disease Advisory Councils (RDACs) – which exist in 27 of the 50 states in the United States. GLI’s Pediatric and Rare Liver Diseases program director Kristin Hatcher has been appointed to the Mississippi Rare Disease Advisory Council Board of Directors, joining council member L. Marie Assad of the Friends of PBC as an RDAC member.
Representing the rare liver diseases patient community at the FDA Innovation Hub
The FDA is offering a unique opportunity for rare liver disease patients and representatives to share their needs during an open, public meeting on October 16, 2024 for the newly announced Rare Disease Innovation Hub. GLI is excited to participate; our community’s input will set the precedent for interaction between regulators, patient advocacy groups, and nonprofit organizations, ultimately influencing decisions on drug pipelines and treatment options for rare liver diseases.
Register to join GLI virtually or in-person in Washington, D.C. No matter the manner of your participation, the attendance by advocates across the breadth of rare liver diseases will demonstrate the strength and unity of our community, which should help ensure our needs are represented and acknowledged.
Advocacy for the John W. Walsh Alpha-1 Home Infusion Act in Mississippi with Congressman Trent Kelly
GLI’s Program Director of Pediatric and Rare Diseases Kristin Hatcher was honored to present The John W. Walsh Alpha-1 Home Infusion Act of 2023 (H.R.4438) with the Alpha-1 Foundation, Hope for PDCD Foundation, and Everylife Foundation. They discussed the effect that this bill has on patients, particularly in rural areas in which patients have to travel long distances to even receive their infusions. The bill’s fiscal responsibility allows rural populations, like Mississippians and Tennesseans, to both access equitable healthcare and save taxpayer money. We look forward to working with Trent Kelly’s office on this important piece of legislation.
Approval for Intellia clinical trials for Alpha-1 Antitrypsin Treatment Option in the UK
The UK’s Medicines and Healthcare Products Regulatory Agency has approved clinical trial applications for NTLA-3001, an in vivo, CRISPR-based therapeutic developed by Intellia Therapeutics for Alpha-1 antitrypsin deficiency (AATD). This genetic disorder impacts both the lungs and the liver in some patients, and the introduction of a one-stop, genetically modifying treatment represents a significant advancement.
NTLA-3001’s gene editing aims to eliminate the need for weekly AAT augmentation therapy and reduce the need for lung transplants, offering hope for a transformative change in comprehensive patient care.
Delivering Livdelzi® quickly to PBC patients needing second-line therapy
Gilead Sciences and CymaBay Therapeutics have recently received accelerated approval for seladelpar (Livdelzi®) for PBC – a valuable new treatment option for patients. Seladelpar, which has received accelerated approval from the U.S. Food and Drug Administration, is now accessible at pharmacies across the US. It offers a second-line treatment for the approximately 40% of PBC patients who do not respond The swift availability of this new option is an important step forward for patients seeking effective management of their condition.
Amplify the message for International PBC Day on September 8, 2024
International PBC Day is approaching, and there’s an opportunity to make a meaningful impact. The PBC Foundation has made significant strides by providing graphics in a range of languages to raise awareness about PBC and emphasize the importance of open discussions. GLI is eager to collaborate with PBC patients and organizations to drive crucial dialogue and awareness on a global scale. Let’s use this day to highlight the challenges of PBC and advocate for better understanding and support!
BARE Connection Network is looking for patients and families with biliary atresia
We are excited to help promote the BARE Connection Network, a vital initiative by BARE Inc to connect patients with biliary atresia (BA) to supportive communities and families. Per BARE Inc’s description online, “[t]he BCN is [its] proactive step towards fulfilling this crucial need, providing a dedicated platform where families can come together to share, support, and strengthen each other.”
If you are a BA patient in Alabama, Delaware, Hawaii, Maine, Rhode Island, South Dakota, and Vermont, the BA community is looking for you to join!
Possible new biomarker for early detection of biliary atresia
A multicenter study in Japan has identified urinary oxysterols, particularly 27-hydroxycholesterol, as a promising biomarker for detecting BA in neonates. By distinguishing BA from other causes of neonatal cholestasis, this research may lead to more timely interventions, and thus better outcomes for patients and their families. However, additional studies are needed to confirm these findings and ensure their reliability before they can be applied at the bedside.
Upcoming Events
Upcoming GLI events:
- September 13 – 16, 2024 – GLI’s Advanced Advocacy Academy (A3)
Other events:
- September 9 – 12, 2024 – C-Path’s Premier Global Impact Conference
- September 25 – 28, 2024 – Global Genes Week in RARE
- September 26 – 27, 2024 – RARE Advocacy Summit
- September 28, 2024 – RARE Health Equity Forum
- October 4 – 6, 2024 – Wilson Disease Association Annual Conference
- October 18 – 20, 2024 – PSC Partners Annual Conference
- October 20 – 22, 2024 – NORD Breakthrough Summit
- November 6 – 9, 2024 – NASPGHAN Annual Meeting
- November 15 – 19, 2024 – AASLD The Liver Meeting 2024
For more information about the Pediatric and Rare Liver Diseases Council or to learn more about joining, please visit our webpage or email pedsrare@globalliver.org.