
How to Stay Engaged Beyond Rare Liver Diseases Month – Pediatric and Rare Liver Diseases News
#RareAware Campaign: Our Impact on Rare and Rural Healthcare
In February, we took action to bridge healthcare gaps for rare liver disease patients in rural communities—driving awareness, advocacy, and education where it’s needed most. Here are some highlights:
- Our February episode of Is Your Liver Healthy? podcast brought together Dr. Harsha Rajasimha of the US Indo Rare Disease Foundation, Dr. Cecilia Dueñas of the PBC Research Foundation, and Theresa Davidson of the MidSouth Liver Alliance to discuss the urgent need for equitable access to clinical trials for rural and rare patients around the globe.
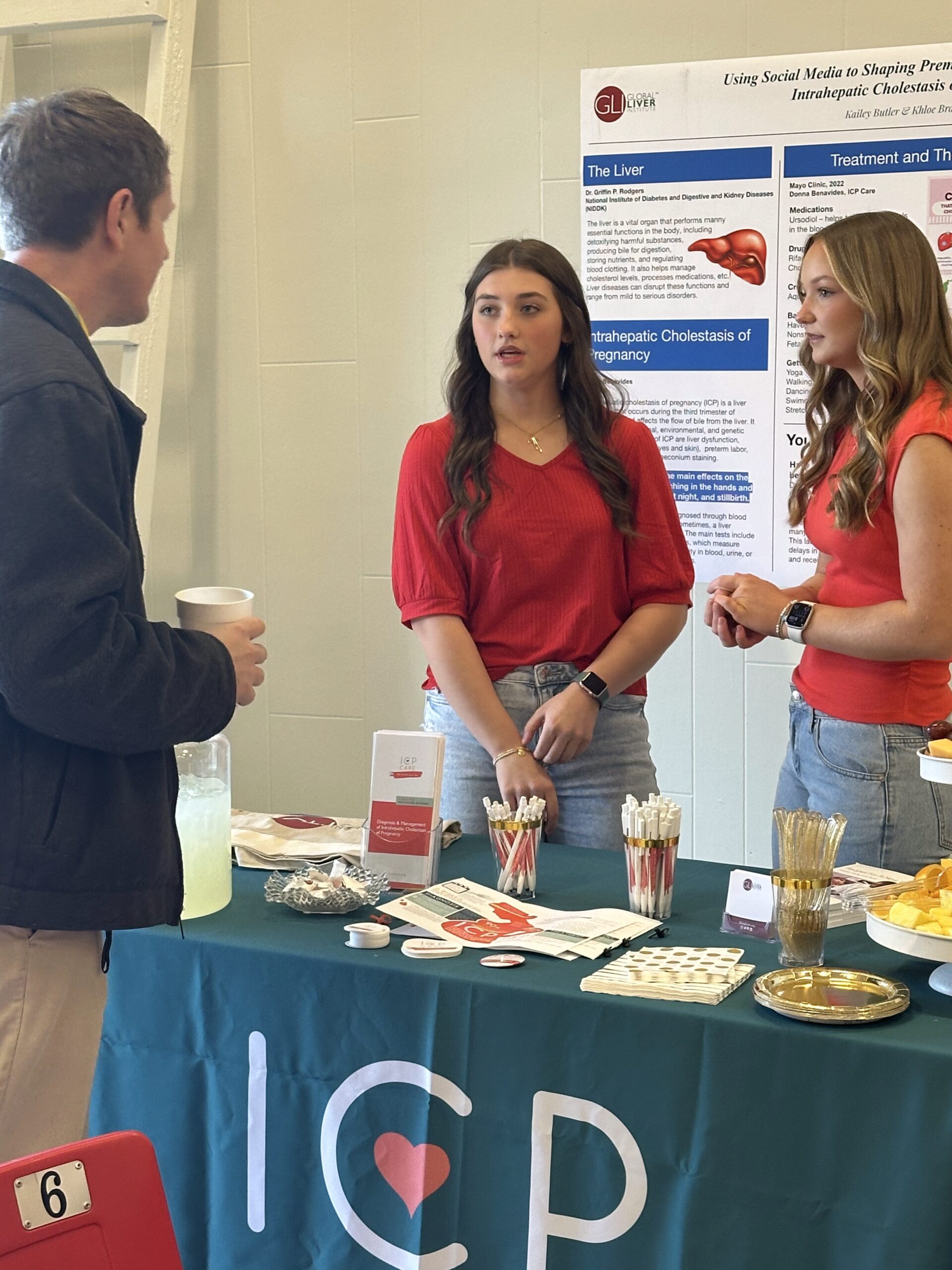
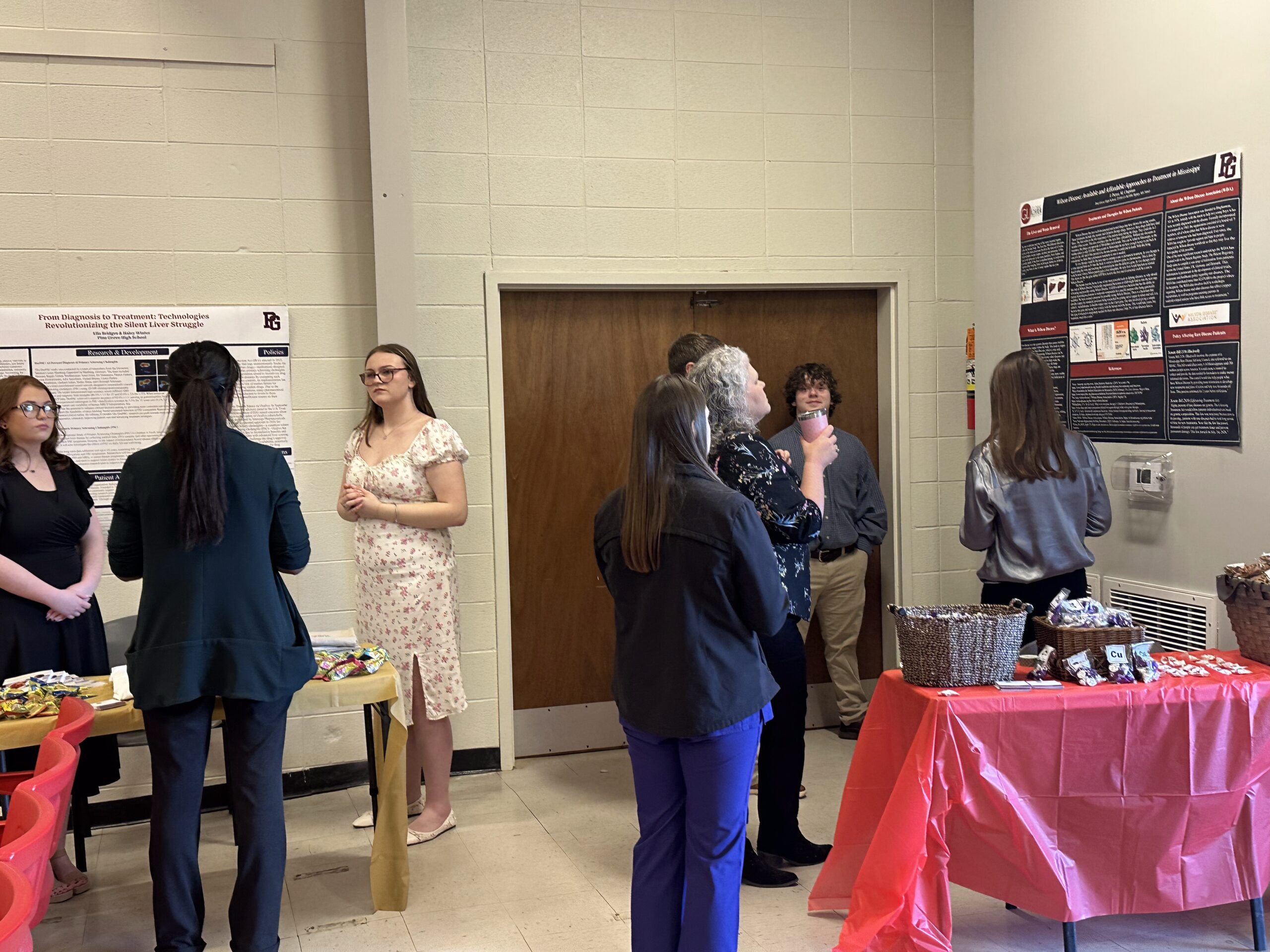
- In the community, our pilot Rare Science Fairs program in Mississippi reached over 500 individuals, featuring seven research posters co-created with students and patient advocacy leaders to spark local engagement in rare disease research.
- On Capitol Hill, we championed the ORPHAN Cures Act, a critical bipartisan bill that protects incentives for developing rare disease treatments by ensuring orphan drugs remain exempt from Medicare price negotiations if they are approved for more than one rare condition.
But the work doesn’t stop here. There are still many ways to engage beyond Rare Liver Diseases Month. Here are some ways to engage:
- Direct patients and caregivers to our newly released rural healthcare resources, now available on our website.
- Help us grow our Rare Science Fairs and bring them to more schools worldwide.
- Urge your representatives to support policies that expand access to rare disease care. The ORPHAN Cures Act still needs backing—use this resource to make your voice heard.

New Key Screening Questions for Early Diagnosis of Biliary Atresia
Early detection of biliary atresia is critical for timely intervention, yet diagnoses are often delayed. A new AAP guidance clinical report emphasizes three key questions for primary care providers: Is the infant jaundiced, do they have pale stool, was their direct bilirubin high at birth? If the answer to any of these is yes, a direct bilirubin level should be checked to assess for possible liver disease.
Routine screening in primary care settings can significantly improve early diagnosis and outcomes for affected infants. Collaboration with pediatric hepatologists and awareness of patient advocacy resources can further support families navigating this condition.
A Key Reason for Misdiagnosis of Alagille Syndrome in Low-Resource Settings
A recent publication from South Africa highlights the challenge of diagnosing alagille syndrome (ALGS) in settings with limited access to genetic testing, where it is often misdiagnosed as biliary atresia. Without proper diagnostic tools, children may undergo unnecessary surgeries, as early liver biopsy findings can resemble biliary atresia. This publication underscores the urgent need for better diagnostic strategies, education, and resource allocation to prevent misdiagnoses, particularly in low-income regions.
FDA Grants Chemomab Phase 3 Trial Approval for Nebokitug in PSC
Progress is being made in the treatment of Primary Sclerosing Cholangitis (PSC) as the FDA has granted Chemomab approval to proceed with a Phase 3 trial for Nebokitug. PSC is a chronic liver disease that causes inflammation and scarring of the bile ducts, leading to serious complications like liver failure and cancer. With no approved treatments, patients rely on symptom management and, in severe cases, liver transplants. Nebokitug targets the underlying disease, aiming to slow progression and improve outcomes. Notably, this Phase 3 trial evaluates clinical outcomes without requiring liver biopsies—a meaningful shift from traditional PSC study designs.
Highlighting the Fight for Life Club in Mexico
The National Institute of Pediatrics (INP) and the Fight For Life Club Foundation have achieved a significant milestone in pediatric transplantation, successfully performing two living donor liver transplants in a single week. With only 4.5 deceased organ donors per million people in Mexico, living donor transplants are a critical solution to reducing wait times and improving survival rates for children. This initiative is part of a broader effort to build a sustainable, world-class pediatric transplant program in Mexico.
Expanded Newborn Screening in Victoria Enhances Early Detection of Rare Diseases
Galactosemia, a rare metabolic disorder affecting approximately 1 in 50,000 newborns, can lead to life-threatening liver disease if left undiagnosed. Early detection through newborn screening is critical to preventing serious complications and enabling timely intervention. Victoria, Australia, has expanded its newborn screening program to include galactosemia, bringing the total number of screened conditions to 32.
Recognizing Life-Saving Liver Transplants in Hyderabad, India
Osmania General Hospital (OGH) has made a remarkable impact by successfully performing five liver transplants in a month, providing life-saving treatment to young patients from low-income backgrounds and treating patients with Tyrosinemia Type 1, Wilson’s disease, and Chronic Budd-Chiari syndrome. These complex procedures, typically costing 3 to 4 million rupees in private hospitals, were performed free of charge under the state’s Aarogyasri scheme, ensuring access to critical care for those who need it most. This achievement highlights OGH’s growing excellence in liver transplantation, particularly for rare liver diseases.
Conditional Marking Approval for Seladelpar in Europe
Seladelpar has received conditional approval in Europe for the treatment of primary biliary cholangitis (PBC), either in combination with UDCA or as monotherapy for those unable to tolerate UDCA. This approval expands much-needed treatment options for PBC patients, a condition with limited therapeutic choices. While conditional approval allows early access, additional confirmatory trials are required to fully establish its clinical benefits.
Upcoming Events
- March 14 – 15, 2025 – GLI’s Advanced Advocacy Academy, London, United Kingdom
- March 20 – 22, 2025 – Liver Connect, San Antonio, Texas, USA
- March 24 – 25, 2025 – 16th International Conference on Liver Diseases & Hepatology, London, United Kingdom
- April 4 – 5, 2025 – 2025 Alpha-1 Foundation 7th Global Research Conference and 10th Patient Congress, Lisbon, Portugal
- April 8 – 11, 2025 – International PBC Summit 2025, Edinburgh, Scotland
- April 22 – 24, 2025 – World ORPHAN Drug Congress USA 2025, Boston, Massachusetts, USA
For more information about the Pediatric and Rare Liver Diseases Council or to learn more about joining, please visit our webpage or email pedsrare@globalliver.org.
