
Fatty liver disease (FLD) and nonalcoholic steatohepatitis (NASH) are increasing in prevalence worldwide, creating a major global public health crisis. To adequately educate patients, practitioners, and policymakers, there is a need to collect, curate and share relevant information. NASH News, published on behalf of the Global Liver Institute’s NASH Council, intends to meet that need and to facilitate collaboration across the emerging NASH community on a monthly basis.
We would appreciate your feedback and content contributions. Please contact nash@globalliver.org
In 2020, GLI and the GLI NASH Council released the U.S. NASH Action Plan to comprehensively address NASH and its impact on patients and families, public health, and the economy. The recommendations presented in the U.S. NASH Action Plan were developed to address critical actions necessary to stop the rise of this life-threatening disease. Issues include a lack of awareness and education, lack of agreement on how to diagnose, lack of standardized patient management and treatment for NASH, and a lack of NASH-specific policy initiatives. Since the U.S. NASH Action Plan’s creation, the liver community has worked collaboratively to move the field forward and elevate conversations surrounding NASH. As we approach the end of 2021, we take a moment to recognize and highlight the achievements of our NASH Council members, stakeholder groups, and affiliate partners who have stepped up to address these issues.
Throughout 2021, GLI’s NASH Council member organizations worked tirelessly to promote advancement in NASH. We have witnessed the discovery of new therapeutic solutions for lifestyle management such as the development of the GLI nutrition app (with NutriStyle). The American Gastroenterological Association worked with the American Diabetes Association, American Osteopathic Association, Endocrine Society, and the Obesity Society on their development of a NAFLD/NASH Clinical Care Pathway. The European Association for the Study of Liver Disease updated guidelines on the use of non-invasive techniques.
GLI hosted the first NASH Externally-Led Patient Focused Drug Development meeting for the FDA. Stepping up to address the lack of NASH treatment availability, GLI applauds the actions of the FDA to grant breakthrough device designation for Orbera® Intragastric Balloon (IGB) as a treatment for NASH. Through the creation of patient advisory boards, our NASH Council members, Boehringer Ingelheim and Pfizer Inc. fostered a collaborative discussion with GLI-identified patients to ensure their perspectives were incorporated into design. There has been unprecedented momentum gained in the NASH policy realm as GLI continued to work with partners and patient advocates in the liver community to advance policy supporting liver patients through the reintroductions of The LIVER Act, The NASH Care Act, and The Recognizing October as Liver Cancer Awareness Month Resolution.
Only together we can achieve victory for patients and caregivers who are impacted by this progressive, deadly disease.
Read more about our accomplishments in the U.S. NASH Action Plan 2021 Scorecard.
Donna R. Cryer, JD
President & CEO
Global Liver Institute
GLI News

GLI Led First-Ever NASH Externally Led Patient-Focused Drug Development (EL-PFDD) Meeting
On November 4th, GLI, in collaboration with the GLI Liver Action Network (LAN), led the first-ever EL-PFDD Meeting on NASH. This virtual event, viewed by a global audience, included two panels of patients and caregivers impacted by NASH. The successful meeting provided the FDA, as well as the medical community, an opportunity to obtain a wide range of patients’ and caregivers’ input on NASH, including their perspectives on their condition, its impact on daily life, and the urgency around developing therapies. Read more in our Patient Perspective section below.
If you have been impacted by NASH, the GLI LAN and GLI invite you to participate in this important survey to help develop future treatments for NASH. The results from this survey will be included within the final EL-PFDD outcome report. Take the survey
Global Liver Institute Recognized with Two Advocacy Awards for Enduring Contributions and Sustained Service


GLI President & CEO Donna R. Cryer, JD, is the first-ever, proud recipient of the AASLD “Distinguished Advocacy Service Award” at the 2021 Liver Meeting. The Distinguished Advocacy Service Award is given to an advocacy organization or individual in honor of sustained service to the liver disease community in general. The award recognizes service provided to the hepatology community over an extended period that raises awareness or garners public and federal legislative support and promotes liver health and quality patient care.
The RARE Champions of Hope Founder’s Award — which Mrs. Cryer received a week later on November 18 at a ceremony that was part of the Global Genes RARE Health Equity Summit in Philadelphia, Pennsylvania — recognizes founders of impactful organizations in the rare disease community and acknowledges a commitment to connecting, empowering, and inspiring the community and advocating for people living with a rare disease.
GLI Staff Representation Throughout AASLD The Liver Meeting Digital Experience (TLMdx)
Grit, Grace, Gratitude, and Resilience: What You Wish Your Doctors Knew About You with Donna Cryer, JD, GLI Founder and CEO
At this session, Donna R. Cryer, JD, Founder, President and CEO of GLI, used elements from her personal patient and extensive professional journey to illustrate how we can collectively move towards acknowledging the patient within the person and the person with the patient, expand on the power of purpose and the purpose of power, and elaborate on the seven habits of highly effective patient leaders.

Liver Action Network – The Evolution of Community Liver Advocacy with Andrew Scott, GLI Policy Director
At this community conversations session, Andrew Scott, GLI Policy Director talks about how the Liver Action Network (LAN) acts as a first-of-its-kind affiliation model that facilitates provision of strategic mentorship, technical assistance, to LAN Roundtable Member Organizations, and affords a central structure for the formulation of policy positions and programmatic solutions by advocates, whether patients, family members, caregivers, or providers. Community members had the opportunity to meet with LAN members in this interactive discussion.

Abstract Tour: Pediatrics & Rare Disease with Robert Mitchell-Thain, GLI Pediatric and Rare Liver Diseases & Patient Insights Director
At this patient forum, Robert Mitchell-Thain, GLI Pediatric and Rare Liver Diseases & Patient Insights Director, led the patient community on a virtual tour through some of the abstracts in the areas of pediatrics and rare liver diseases presented at this year’s TLMdx.

NASH Summit 2021
For the 5th year, the NASH Summit brought cutting edge insights and thought-leading discussion to advance the front line of NASH candidate success. As the world’s largest gathering of NASH drug developers, this groundbreaking digital industry-specific discussion and networking forum is the unrivaled conference of the NASH biopharma calendar. GLI’s NASH Programs Director, Jeff McIntyre, presented at this year’s virtual event.

Surfing the NASH Tsunami
Every week, a global community of fatty liver disease stakeholders comes together to explore the most important challenges in diagnosing, treating, and developing medications for patients. Catch the latest episodes of Surfing the NASH Tsunami podcast featuring members of GLI staff.
- Go Inside a Pivotal Event: The NASH Patient-Focused Drug Development Meeting featuring Donna Cryer, GLI President and CEO, and fellow patient advocate Terri Milton as they review the ground-breaking NASH EL-PFDD meeting held on November 4, 2021.
- Day Three at the 2021 TLMdx from AASLD featuring GLI NASH Programs Director, Jeff McIntyre, Profs. Scott Friedman and Michelle Long, and Dr. Naim Alkhouri join Louise Campbell and Roger Green to review some of the most important and exciting presentations from the first three days of the 2021 TLMdX.
- Day Four at the 2021 TLMdx from AASLD featuring GLI NASH Programs Director, Jeff McIntyre, Drs. Michael Charlton, Mazen Noureddin and Stephen Harrison join Louise Campbell and Roger Green to review some of the most important and exciting presentations from the final two days of the 2021 TLMdX.

GLI LIVE
Join GLI President and CEO, Donna Cryer in conversation with world experts in policy, research, clinical care, and wellness as they put liver patients and the challenges they face front and center. Join GLI LIVE with your questions on Facebook, Twitter, or GLI’s YouTube channel every Wednesday at 12 PM ET.
Upcoming GLI Events
December 16, 2021: NASH Council Meeting. Contact NASH@globalliver.org
pre-wrap;>January 20, 2022: Beyond the Biopsy (en français). Contact NASH@globalliver.org
June 9, 2022: International NASH Day. Contact Livia Alimena for more information
Patient Perspective
Patient Experiences Shared at First-Ever NASH EL-PFDD Meeting
On November 4, 2021, GLI was joined by members of the liver advocacy community to provide their input on NASH, including their perspectives on their condition, its impact on daily life, and the urgency around developing therapies to the U.S. Food and Drug Administration (FDA).
“Is NASH a benign disease?” asked Donna Cryer, JD – an important question posed to the patient panel.
Anthony: “It is far from a benign disease”.
Megan: “It is not silent at all. It is silent in the way that it does not present in the same way.”
John: “A medical student said, oh that’s not a big deal”. “It is something we need to pay attention to and monitor and treat.”
Terri: “When people say it is not serious…they see me on my good days, and not my challenging days. It is a challenge. It is not a benign condition in any form. It is a killer.”
The NASH EL-PFDD Meeting is a critical first step to move the field of NASH forward, as it provides the medical community with a deeper understanding of the impacts of NASH from a patient and caregiver perspective. Watch the testimonies.
GLI Partner Highlight

Mid South Liver Alliance
GLI is pleased to welcome Mid South Liver Alliance as one of the newest members of the GLI NASH Council. Recognizing the need in the Mid-South to support people with liver disease, the Mid South Liver Alliance was formed to provide education for patients and their families and to sponsor advocacy efforts to target funding and research by working with political leaders.
Global NASH Updates
Referral Care Paths for Non-Alcoholic Fatty Liver Disease-Gearing up for an Ever More Prevalent and Severe Liver Disease
It is currently estimated that over 25% of Europeans have NAFLD. The unnoticed progression of NAFLD and limited awareness among health care professionals both lead to over‐referrals and under-diagnosis. Despite the rising prevalence and severity of NAFLD, there is still much room for improvement among health care professionals across the lines of care and also on the national guideline level. According to EASL‐EASD‐EASO guidelines, non-invasive tests (NITs) should aim to identify and assess NAFLD in individuals with increased metabolic risk in primary care. Read the review.
EASL Published Nonalcoholic Fatty Liver Disease: Patient Guidelines
EASL recently published a patient guideline intended for all patients at risk of or living with NAFLD. NAFLD is the most frequent chronic liver disease worldwide and comes with a high disease burden. This guide summarizes the current knowledge relevant to NAFLD and its management. It has been developed by patients, patient representatives, clinicians, and scientists and is based on current scientific recommendations, intended to support patients in making informed decisions.
Research & Development
Lipocine Announces FDA Grants Fast Track Designation to LPCN 1144 for Treatment of Non-Cirrhotic NASH
Lipocine Inc., a clinical-stage biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to LPCN 1144 as a treatment for non-cirrhotic NASH. The FDA’s Fast Track Designation is designed to accelerate the development and expedite the review of products that are intended to treat serious diseases and for which there is an unmet medical need.
Association of Bariatric Surgery With Major Adverse Liver and Cardiovascular Outcomes in Patients With Biopsy-Proven Nonalcoholic Steatohepatitis
NASH is rapidly becoming the leading cause of cirrhosis and hepatocellular carcinoma and is also significantly linked to cardiovascular disease. When lifestyle modifications are no longer sufficient, bariatric surgery may be considered to assist patients with weight loss. In this retrospective cohort study of 1158 patients, researchers found that among patients with NASH and obesity, bariatric surgery was significantly associated with lower risk of major adverse liver outcomes as well as cardiovascular events.
GLI NASH Resources

NAFLD/NASH: How Can Patients Participate in Clinical Trials
GLI recently released a guide to participating in clinical trials for patients with NAFLD/NASH. NAFLD/NASH: How Can Patients Participate in Clinical Trials is a comprehensive resource that provides answers to your questions about clinical trials for NAFLD/NASH patients and caregivers – what they are, why they are important, questions to ask, and where to find them. Coming soon in French and Spanish.
NASH Core Curriculum: A Comprehensive Online Resource Center

GLI, in collaboration with Clinical Care Options, launched the CME course, NASH Core Curriculum: A Comprehensive Online Resource Center to improve clinician understanding of foundational concepts in NASH diagnosis, management, and emerging pharmacologic treatment strategies. Resources are developed primarily for clinicians, including advanced practice clinicians, in hepatology, gastroenterology, endocrinology, and primary care. The full curriculum includes CME/CE-certified video modules, ClinicalThought™ expert commentaries, and downloadable presentations and resources.
The most recent module shared online focuses on Designing the Ideal NASH Therapy and features Stephen Harrison, MD, FACP, FAASLD, COL (ret.) USA, MC, as the presenter. NASH Core Curriculum is supported by educational grants from Gilead Sciences and Novo Nordisk Inc.
U.S. NASH Action Plan: Recommendations for Payors

GLI and the GLI NASH Council released the U.S. NASH Action Plan to comprehensively address NASH and its impact on patients and families, public health, and the economy. It includes a set of actionable recommendations for the full spectrum of groups involved in NASH prevention and treatment. This month, we’re highlighting some of the recommendations for payors:
- Education: Engage patient advocates and patient advocacy organizations across the spectrum of NAFLD/NASH and with a diversity of common comorbidities to understand the impact and integrated care needs of patients.
- Diagnosis: Reimburse for non-invasive diagnostics to make safer, cost-effective screening and treatment response methods accessible to more clinicians and patients.
- Patient Management/Treatment: Reimburse (across plan types) for integrated, whole person care including evidence-based NAFLD/NASH-related services such as dieticians, exercise specialists, weight loss medications, and bariatric surgery.
- Policy Effort/Legislation: Collaborate with medical societies to conduct a study within medical insured populations prior to setting budgets/internal policies.
GLI Nutrition App with NutriStyle
GLI and NutriStyle Inc. have partnered to create a personalized nutrition app for people living with liver disease, diabetes, and other chronic conditions or who want to maintain good liver health. The app will create personalized meal plans to meet the specific requirements set out by GLI nutritional advisors for people with NASH or a general interest in liver health. Visit NutriStyle to learn more.
Reaching At-Risk Patients Through COVID-19 Vaccination Sites
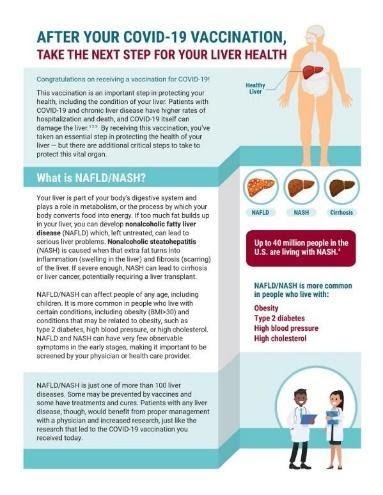
GLI launched a collaborative effort with Summit Clinical Research to promote NASH awareness in the context of liver health and COVID vaccination via partnerships through city vaccination sites and direct patient education. Providers will be giving people who have just received their COVID-19 vaccination our new resource, After Your COVID-19 Vaccination, Take the Next Step for Your Liver. Please contact NASH@globalliver.org if you would like to share this resource through a COVID-19 vaccination site.
Clinical Care
Economic and Clinical Burden of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes in the U.S.
NASH is strongly associated with type 2 diabetes mellitus (T2DM). Patients with both T2DM and NASH have increased risk for adverse clinical outcomes, leading to higher risk for mortality and morbidity. Additionally, NAFLD and NASH are associated with significant health care utilization. This is the first study to assess both the economic and clinical burden of NAFLD and NASH in patients with T2DM. Read the study.
Food Concerns Linked to Liver Disease
New research has highlighted the importance of food insecurity as a contributing factor to outcomes in U.S. adults with NAFLD and advanced fibrosis. “Food insecurity…has emerged as an important social determinant, one associated with a greater risk of NAFLD and NAFLD-associated liver fibrosis,” says Dr. Ani Kardashian, an assistant professor of clinical medicine at the Keck School of Medicine of USC. Read the database analysis.
Current Clinical Trials
Clinical trials are at the heart of all medical advances. The goal of clinical trials is to determine if a new test or treatment works and is safe as well as other aspects of care, such as improving the quality of life for people with NAFLD or NASH. Since there are currently no medications approved for the treatment of NAFLD or NASH, clinical trials offer hope for many people and an opportunity to help researchers find better treatments for others in the future.
