During the Congressional recess in August, GLI prepared for a busy fall. Congress returns in September to advance appropriations bills and debate key legislative priorities. The Administration will continue its work related to Medicare coverage policy, FDA consideration of new drugs and devices, and advancing an NIH research agenda.
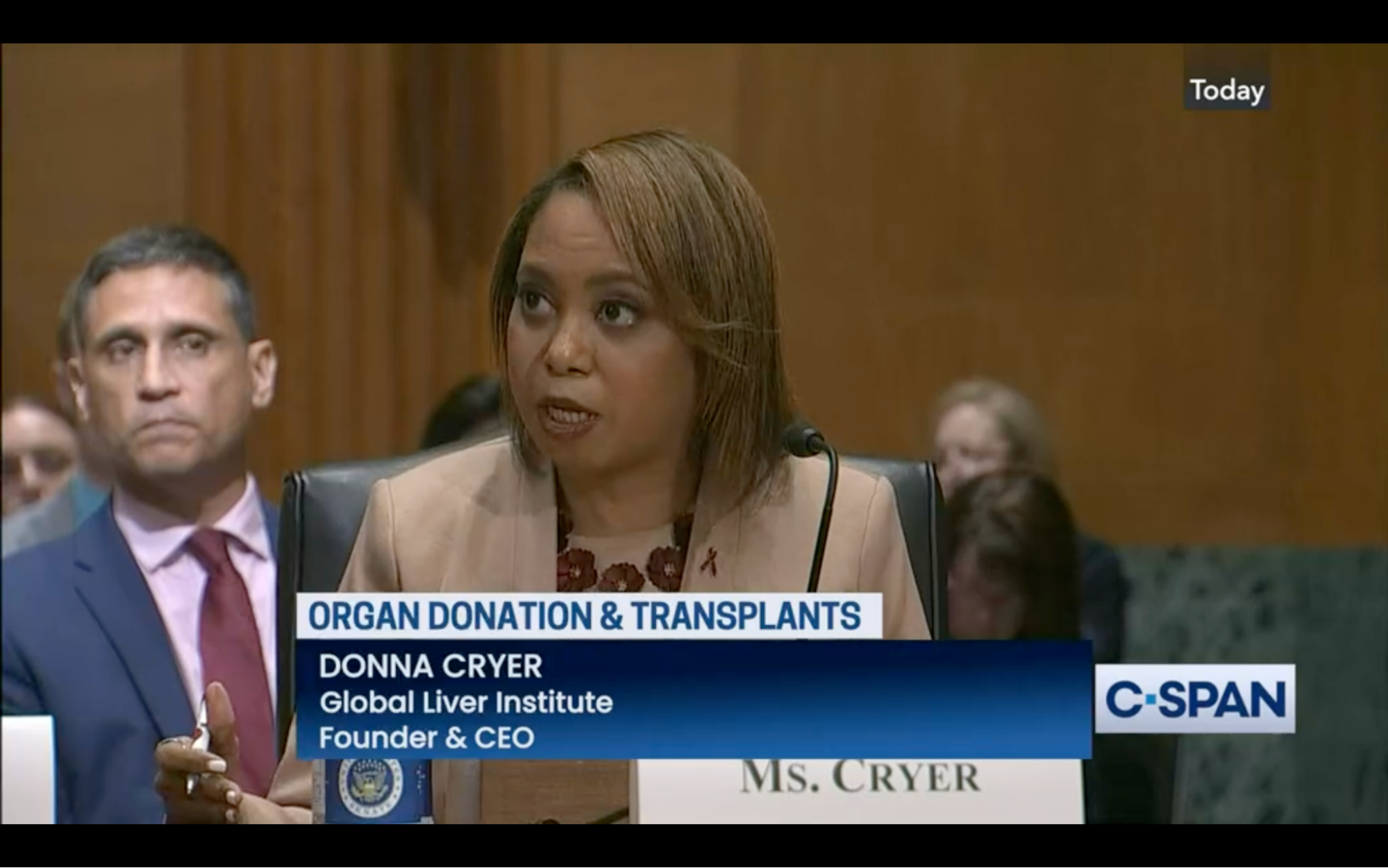
HRSA Responds to passage of bipartisan legislation reforming the organ donation system after GLI testified in person at the Senate Finance Committee on July 20, 2023.
GLI CEO Donna R. Cryer, JD, testified at a Senate Finance Subcommittee on Health Care hearing titled “The Cost of Inaction and the Urgent Need to Reform the U.S. Transplant System.” After the hearing, bipartisan legislation responding to the urgent need for reforming the U.S. Organ Procurement and Transplantation Network (OPTN) was passed by Congress. Health Resources and Services Administration (HRSA) Administrator Carole Johnson released a statement following passage of the bill. She stated that HRSA shared Congress’ goal of improving and strengthening the OPTN, commended bipartisan leaders in the House and Senate, and committed to quickly implementing the policy changes. GLI will be following the bill’s implementation and HRSA’s OPTN Modernization Initiative closely to ensure policymakers are remaining accountable for advancing its goals.
Congressional debate on appropriations bills will be a focus in Congress in September.
For the first time in 5 years, all Senate appropriations bills were introduced and passed by the Senate Committee on Appropriations in a bipartisan fashion, with leadership from Chairwoman Patty Murray and Ranking Member Susan Collins. The bill provides modest increases for key agencies and health programs such as NIH, CDC and DOD. To learn more about the specific funding levels in the Senate bill, please reference the draft report accompanying the Senate Labor, Health and Human Services bill. The House Labor, Health and Human Services bill does not include a draft report to accompany the bill that was passed on a partisan vote by the House committee. Spending cuts in the House largely came from health-related programs and are anticipated to be the source of significant debate in Congressional efforts to fund the government beyond this fiscal year, which ends September 30, 2023.
NIDDK’s Advisory Council to meet on September 13, 5 council seats expire at the end of year.
The next meeting of the NIDDK’s National Diabetes and Digestive and Kidney Diseases Advisory Council (NDDKAC) will be held on September 13 at the NIH Main Campus in Bethesda. With 5 council seats expiring at the end of the year, GLI is pleased to nominate Julius M. Wilder MD, PhD to be a member of the NDDKAC. Not only is Dr. Wilder an accomplished gastroenterologist, transplant hepatologist, and medical sociologist with a national reputation in health equity, he would bring much-needed diversity to the NIH NIDDK Council’s work. View the nomination supported by GLI and other leading organizations.
GLI has endorsed several key pieces of legislation that may be considered in the fall.
GLI looks forward to working with its partners to pass the following bills:
- Treat and Reduce Obesity Act, legislation introduced by U.S. Senators Tom Carper (D-Del.) and Bill Cassidy (R-La.) and Representatives Raul Ruiz (D-Calif.) and Brad Wenstrup (R-Ohio) to combat the obesity crisis – access the Obesity Action Coalition’s Action Center to urge cosponsors.
- Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act, legislation creating a benefit category for MCED tests – access Prevent Cancer Foundation’s Action Center to urge cosponsors.
- Living Donor Protection Act, legislation protecting living donors – access the AAKP’s Action Center to urge cosponsors.
- Saving Access to Laboratory Services Act (SALSA), legislation supporting access to testing and innovation – access the Action Center to urge cosponsors.
- Hepatitis C Elimination Program, program proposed in the President’s budget for FY 2024 – GLI is following closely for legislation to be introduced advancing the program.
GLI requests input from government entities, hepatology and larger healthcare and health policy communities on the impact of and response to AASLD’s nomenclature change for nonalcoholic fatty liver disease (NAFLD).
Last September, GLI’s CEO Donna R. Cryer, JD, provided insights on proposals to change the name of NAFLD and NASH to eliminate the term “non-alcoholic” by noting that this debate did not start with patients nor did it respond to patient priorities. Following the official publication of changes despite patient concerns, GLI is leading an impact analysis to mitigate confusion among patients and clinicians and understand the associated costs to patient advocacy and regulatory approval alike. We welcome your feedback on steps your organization will take to respond to these changes. Please email nash@globalliver.org to join the next meeting or direct your input.
A3: The Learning Experience
Washington DC, September 30 – October 2, 2023
Liver patients, their loved ones, and clinician advocates will be gathering in Washington, DC, at the end of the month to gain critical knowledge and skills for effective liver patient advocacy! Experts in the following topics will equip advocates to effect change in their communities: liver health basics, techniques in media and storytelling, the research and development process, health insurance and coverage, collaboration with policymakers, and more!