Congress continues struggle to advance funding bills, agencies continue their work.
Join Global Liver Institute to Push Congress to Advance Priority Bills and Support Agency Efforts.
The government narrowly averted a shutdown but work continues to advance FY 2024 appropriations bills to keep the government funded.
The Continuing Resolution that keeps the government funded will expire on November 17, 2023. Congress will need to either pass another Continuing Resolution or pass the Fiscal Year 2024 appropriations bills required to fund the government for the full year. GLI continues to support funding levels reflected in the Senate version of appropriations bills, providing modest increases to key health care programs and activities. Please join us by reaching out to your legislators through our easy-to-use form!
New Congressional Support after GLI Advanced Advocacy Academy’s (A3) Meetings

After advocates met with almost 60 Congressional offices to share their shared personal stories, Members of Congress and their staff walked away with a better understanding of how legislation directly impacts people with liver disease and liver cancer, creating momentum for advancing GLI’s legislative priorities. Several signed onto cosponsor key pieces of legislation since the meetings. We are grateful to Congressman Mike Levin for cosponsoring the Living Donor Protection Act, Senator Hickenlooper for supporting the Medicare Multi-Cancer Early Detection Screening Coverage Act, and Senator Thomas Tillis for supporting all four priority bills!
Key Mannatt report discusses obesity treatment and efforts to improve CBO score-keeping.
Manatt Health, with contributions from the Obesity Action Coalition and The Obesity Society (both of which GLI is a member), summarized the legal and policy rationales for CMS to alter its interpretation and concluded that a law passed in 2003 does not prevent Part D coverage of anti-obesity medications. The paper called out that obesity is already recognized as a disease by most medical organizations and medical agencies, and treating obesity is not solely a treatment for weight loss, a fact well understood by many patients with liver disease. CMS could choose to identify obesity as a disease and cover these new treatments under Part D. Also, the Congressional Budget Office published a blog stating it “is on the lookout for new research that would enhance its analysis of policies affecting the use of obesity treatments—especially research on the use of anti-obesity medications (AOMs).”
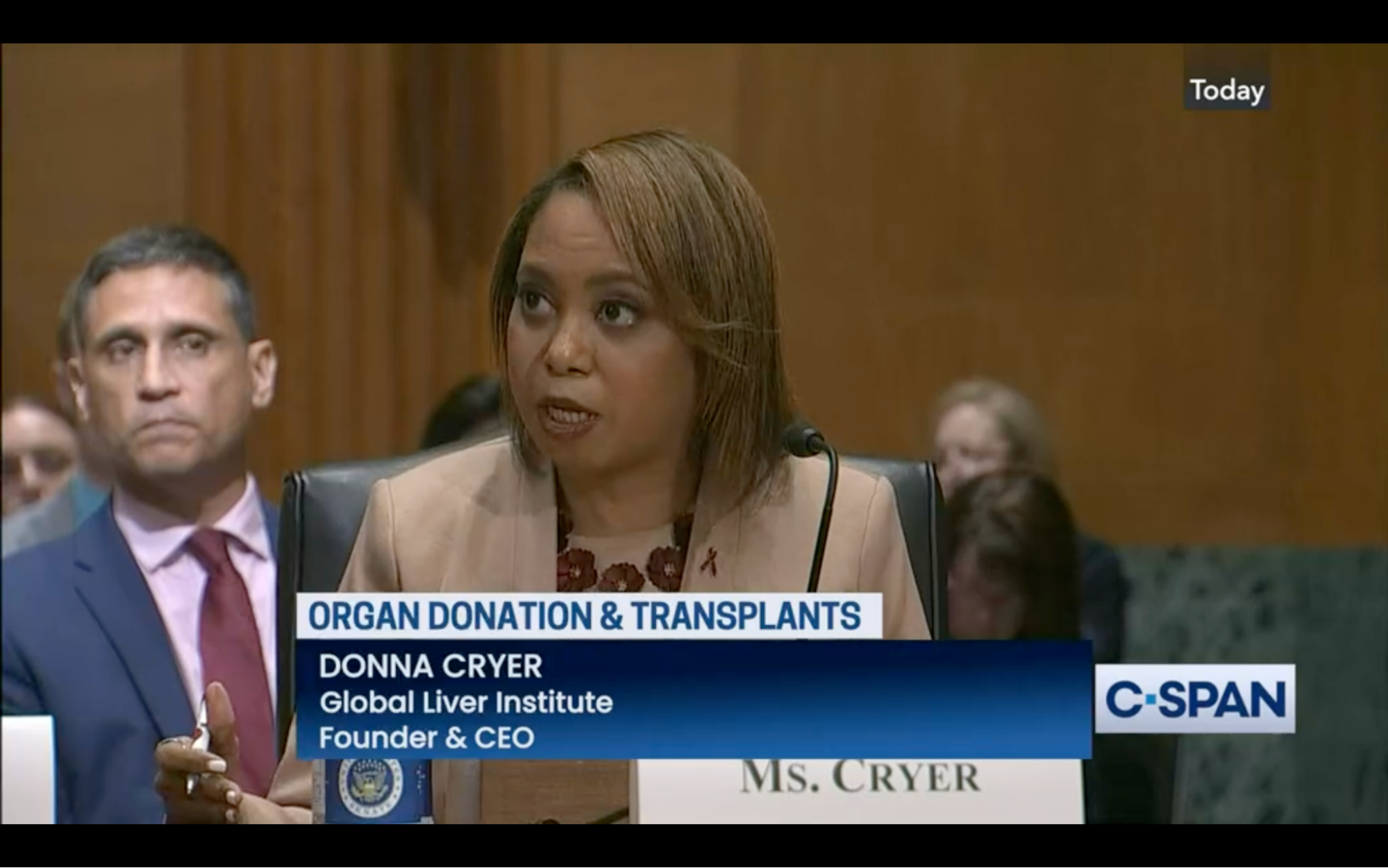
CMS and HRSA engage in coordinated efforts after passage of OPTN Reform Act.
After passing the “Securing the U.S. Organ Procurement and Transplantation Network Act,” which allows for the award of multiple grants, contracts, or cooperative agreements to operate the Organ Procurement and Transplantation Network, CMS and HRSA have committed to drive improvements in donations, clinical outcomes, system improvement, quality
measurement and transparency, and regulatory oversight. The agencies published an announcement about a coordinated effort to improve organ donation, procurement, and transplantation through an Organ Transplantation Affinity Group (OTAG). Also, HRSA responded to calls for improved stakeholder engagement related to the OPTN Modernization Initiative by providing a web-based contact form, now available on the OPTN Modernization website to solicit feedback from patients, families, clinicians, and other interested parties. GLI was proud to be represented at the bill’s signing ceremony, and is pleased to see these continued efforts toward modernization since its passage.
HHS Office for Civil Rights has proposed changes to Section 504 of the Rehabilitation Act impacting access to liver transplants for people with disabilities.
The HHS has taken additional steps to ensure access to organ transplants for all people by removing barriers for people with disabilities. The proposed rule acts on the recommendations of a 2019 study from the National Council on Disability (NCD), finding that individuals with disabilities are often excluded from the transplant process due to inaccurate assumptions about quality of life and post-transplant compliance for those individuals. To this end, the Department highlights the importance of including organ transplantations as part of its broader application of Section 504 requirements in the medical treatment context.